TYPES OF WATER
Hard Water
- Alternatively, lime slurry can be diluted in tanks with at least 15 minutes residence time to allow any softening reactions to reach completion. Lime dosing downstream of filters can result in an increase in the turbidity of the filtered water depending on the lime dose, the proportion of impurities in the lime and the formation of calcium.
- Lime softening, in particular, is the removal of Ca and Mg ions through the addition of addition of lime, Ca(OH) 2. Interestingly, silica (SiO 2), a major constituent of concern for RO membrane fouling, can also be removed through the series of precipitation reactions that occur with lime addition.
- Softening can be achieved by adding lime in the form of limewater, Ca (OH)2, which, in a carbonatation reaction with CO2, forms calcium carbonate precipitate, reacts next with multivalent cations to remove carbonate hardness, then reacts with anions to replace the non-carbonate hardness due to multivalent cations with non-carbonate hardness due to calcium.
Water treatment operations like lime softening is a viable application in Flowsheet ESP. The software tool contains the chemistry and unit operations needed to simulate this and other treatment processes effectively. In a softening-reactor a bed of sand and pellets is floating in the water. By adding chemicals like caustic-soda or lime a reaction is triggered that will fo.
What is Hard Water?
Hard water is usually defined as water which contains a high concentration of calcium and magnesium ions. Measurements of hardness are given in terms of the calcium carbonate equivalent, which is an expression of the concentration of hardness ions in water in terms of their equivalent value of calcium carbonate. Water is considered to be hard if it has a hardness of 100 mg/L or more as calcium carbonate.
Softening is the removal of hardness from water. This is not a required part of the water treatment process since hard water does not have any health consequences. However, hard water is problematic for a variety of reasons. Hard water makes soap precipitate out of water and form a scum, such as the ring which forms around bathtubs. In addition to being unsightly, the reaction of hard water with soap results in excessive use of soaps and detergents. Hard water may also cause taste problems in drinking water and may shorten the life of fabrics washed in hard water. Finally, hard water harms many industrial processes, so industries often require much softer water than is usually required by the general public.
Excessively hard water will nearly always have to be softened in order to protect the water treatment plant equipment and piping systems. At a hardness of greater than 300 mg/L as calcium carbonate, scale will form on pipes as calcium carbonate precipitates out of the water. The scaling can damage equipment and should be avoided.
Sources of Hardness
Hardness generally enters groundwater as the water percolates through minerals containing calcium or magnesium. The most common sources of hardness are limestone (which introduces calcium into the water) and dolomite (which introduces magnesium.) Since hardness enters water in this manner, groundwater generally has a greater hardness than surface water. There are also regional variations in hardness, shown by the map below.
Since they are the two most widespread and troublesome ions in hard water, it is often said that hardness is caused by calcium (Ca2+) and magnesium (Mg2+) ions dissolved in water. However, hardness can be caused by several other dissolved metals as well, including strontium (Sr2+), iron (Fe2+), and manganese (Mn2+). You will notice that all of the hardness-causing ions are divalent cations, meaning that they have a charge of positive two. Metals such as sodium (Na+) and potassium (K+) with a charge of positive one do not cause hardness.
Types of Hardness
As mentioned above, hardness in water is caused by a variety of divalent cations, primarily calcium and magnesium. These cations have a tendency to combine with anions (negatively charged ions) in the water to form stable compounds known as salts. The type of anion found in these salts distinguishes between the two types of hardness – carbonate and noncarbonate hardness.
Carbonate hardness compounds Noncarbonate hardness compounds
Calcium carbonate (CaCO3)
Magnesium carbonate (MgCO3)
Calcium bicarbonate (Ca(HCO3)2)
Magnesium bicarbonate (Mg(HCO3)2)
Calcium hydroxide (Ca(OH)2)
Magnesium hydroxide (Mg(OH)2)
Calcium sulfate (CaSO4)
Magnesium sulfate (MgSO4)
Calcium chloride (CaCl2)
Magnesium chloride (MgCl2
As you can see in the table above, carbonate hardness is caused by metals combined with a form of alkalinity. As you may remember, alkalinity is the capacity of water to neutralize acids and is caused by compounds such as carbonate, bicarbonate, hydroxide, and sometimes borate, silicate, and phosphate. In contrast, noncarbonate hardness forms when metals combine with anything other than alkalinity.
Carbonate hardness is sometimes called temporary hardness because it can be removed by boiling water. Noncarbonate hardness cannot be broken down by boiling the water, so it is also known as permanent hardness. In general, it is important to distinguish between the two types of hardness because the removal method differs for the two.
When measuring hardness, we typically consider total hardness which is the sum of all hardness compounds in water, expressed as a calcium carbonate equivalent. Total hardness includes both temporary and permanent hardness caused by calcium and magnesium compounds.
Hardness Problems
In addition to having different removal methods, carbonate and noncarbonate hardness can cause different problems. Carbonate hardness is the most common and is responsible for the deposition of calcium carbonate scale in pipes and equipment. The equation below shows how this deposition is formed in the presence of heat:
Calcium bicarbonate → Calcium carbonate + Water + Carbon dioxide
Ca(HCO3)2 → CaCO3 + H2O + CO2
In addition to the scale (calcium carbonate) produced, carbon dioxide resulting from this reaction can combine with water to give carbonic acid which causes corrosion of iron or steel equipment.
In contrast, noncarbonate hardness is the culprit in forming soap scum. Noncarbonate hardness reacts with the carbonate alkalinity found in soap and detergents in this reaction:
Calcium sulfate + Sodium carbonate → Calcium carbonate + Sodium sulfate
CaSO4 + NaCO3 → CaCO3 + Na2SO4
Softening Processes
Types of Treatment
There are several types of treatment processes which can be used to soften water. Each type is briefly described below. In later sections, we will discuss the two most commonly used processes – chemical precipitation and ion exchange – in more detail.
In each of the treatment processes, the goal is the same. Softened water should have a hardness of about 80 to 90 mg/L as calcium carbonate. If the water is softened further (as in the ion exchange process) then the hard water must be mixed with the softened water to achieve the desired hardness. Excessively soft water can be nearly as problematic as excessively hard water since it causes corrosion of pipes.
Chemical Precipitation
Softening through chemical precipitation is similar to removal of turbidity by coagulation, flocculation, and sedimentation. There are many variations, but the typical process involves adding lime to raise the pH of water until it is high enough for reactions to occur which prompt hardness compounds to settle out of the water. The equipment used also resembles turbidity removal equipment – lime is added in the flash mixer, the water is flocculated, and then the hardness compounds precipitate out in the sedimentation basin.
As mentioned above, groundwater is more likely to need softening than surface water is. Groundwater also may not need flocculation to remove turbidity, so the softening process can sometimes replace the turbidity removal process. If both turbidity removal and softening are required, then the two processes can occur simultaneously, using the same equipment.
Chemical precipitation using lime will remove carbonate hardness. If soda ash is added as well as lime, both carbonate and noncarbonate hardness may be removed. In either case, chemical precipitation does not remove all hardness from water. The hardness can be reduced as low as 30 to 40 mg/L using chemical precipitation, although the typical goal is 80 to 90 mg/L. We will discuss the chemical reactions which occur in lime-soda ash softening in a later section.
Chemical precipitation is an effective softening process, but it does have some disadvantages. The process requires a lot of operator control to get an efficient result, which may make lime softening too operator-intensive for small treatment plants. The high pH used in lime softening can set colors in water and make them difficult to remove. Finally, lime softening produces large quantities of sludge which can create disposal problems.
Ion Exchange
Ion exchange softening, also known as zeolite softening, passes water through a filter containing resin granules. In the filter, known as a softener, calcium and magnesium in the water are exchanged for sodium from the resin granules. The resulting water has a hardness of 0 mg/L and must be mixed with hard water to prevent softness problems in the distributed water.
Ion exchange softening does not require the flash mixer, flocculation basin, and sedimentation basin required for lime-soda ash softening. In addition, the process does not require as much operator time. Ion exchange softening is effective at removing both carbonate and noncarbonate hardness and is often used for waters high in noncarbonate hardness and with a total hardness less than 350 mg/L.
However, ion exchange softening has its disadvantages as well. The calcium and magnesium in the hard water are replaced by sodium ions, which may cause problems for people with health problems who are not supposed to eat any salt. Softeners have to be backwashed in a manner similar to a filter, and the recharge water, known as brine, can cause disposal problems.
Other Softening Processes
Other processes can be used to soften water, but they are generally expensive and only used in rare circumstances. These alternative processes are listed below.
Reverse-osmosis softening involves water being forced through a semi-permeable membrane. Calcium, magnesium, and dissolved solids are captured while the softened water is passed through the membrane.
Electrodialysis involves passing water between two plates with opposite electrical charges. The metals in the water are attracted to the plate with the negative charge while the non-metals are attracted to the plate with the positive charge. Both types of ions can be removed from the plates and discarded. Electrodialysis is used on very hard water, with a hardness of more than 500 mg/L as calcium carbonate.
Distillation involves the evaporation of water. The evaporated water leaves behind all hardness compounds, softening the water.
Freezing will also remove hardness.
To Soften or Not to Soften
Introduction
Since softening is not a required treatment process, each treatment plant must decide whether or not to soften its water. This decision should be made after carefully weighing the advantages and disadvantages of softening.
Advantages of Softening
Softening will deal with the problems caused by hard water – excessive soap use and scaling being the most troublesome. In addition, depending on the type of softening process used, softening may also aid in other water treatment processes. Softening often removes iron and manganese, reduces tastes and odors, reduces total solids content, and removes radioactivity. The high pH associated with lime softening can aid in disinfection. Finally, when water is stabilized using recarbonation at the end of the lime softening process, corrosion in the distribution system is avoided.
Disadvantages of Softening
Softening processes all carry a certain monetary expense. In addition, softening can cause several other problems.
Lime Softening Chemistry
The high pH associated with lime softening tends to favor the formation of hypochlorite as the dominant free chlorine residual, and hypochlorite is a less powerful disinfectant than other free chlorine residuals. The high pH may also increase trihalomethane levels in the water. If the water is not properly stabilized after treatment, then corrosive water may be produced which will corrode the distribution system.
Ion exchange softening, as noted above, can also cause problems due to the high levels of sodium in the treated water. Both lime softening and ion exchange softening create waste disposal problems.
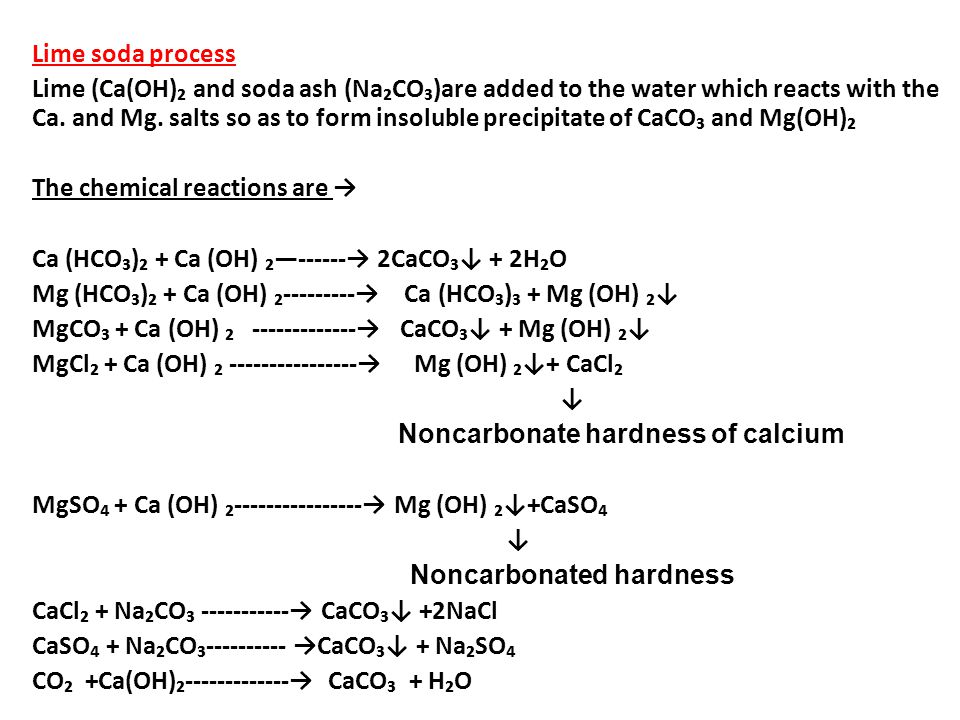
LIME SOFTENING
Lime Softening Chemistry
Introduction
Lime softening involves a relatively complicated series of chemical reactions which will be discussed in depth below. The goal of all of these reactions is to change the calcium and magnesium compounds in water into calcium carbonate and magnesium hydroxide. These are the least soluble calcium and magnesium compounds and thus will settle out of the water at the lowest concentrations. For example, calcium carbonate (which is essentially the same as limestone) will settle out of water at concentrations greater than 40 mg/L.
In order to produce calcium carbonate and magnesium hydroxide, the pH of the water must be raised by the addition of lime. Calcium compounds in water will be removed at a pH of about 9.0 to 9.5 while magnesium compounds require a pH of 10.0 to 10.5. When soda ash is used to remove noncarbonate hardness, an even higher pH is required – 10.0 to 10.5 for calcium compounds and 11.0 to 11.5 for magnesium compounds.
Carbon Dioxide Demand
The first step in lime softening is the addition of lime to water using a typical dry feeder, either volumetric or gravimetric. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Carbon dioxide is the primary compound which creates the initial demand for lime. The following reaction occurs, using up carbon dioxide and lime and creating calcium carbonate and water:
Carbon dioxide + Lime → Calcium carbonate + Water
CO2 + Ca(OH)2→ CaCO3 + H2O
The resulting calcium carbonate precipitates out of solution.
When water, especially groundwater, has a high carbon dioxide concentration, the water is often pretreated with aeration before softening begins. Aeration removes the excess carbon dioxide and lowers the lime requirements.
Removal of Carbonate Hardness
Once the carbon dioxide demand has been met, the lime is free to react with and remove carbonate hardness from the water. Calcium compounds react with lime in the reaction shown below.
Calcium bicarbonate + Lime → Calcium carbonate + Water
Lime Softening Reactions
Ca(HCO3)2 + Ca(OH)2→ 2CaCO3 + 2H2O
We have focussed on calcium bicarbonate since it is the most common calcium compound in water, but other calcium-based hardness compounds have similar reactions. In any case, the calcium carbonate produced is able to precipitate out of solution.
Magnesium compounds have a slightly different reaction. First, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate.
Magnesium bicarbonate + Lime → Calcium carbonate + Magnesium carbonate + Water
Mg(HCO3)2 + Ca(OH)2→ CaCO3 + MgCO3 + 2H2O
Then the magnesium carbonate reacts with lime and creates more calcium carbonate and magnesium hydroxide. Both of these compounds are able to precipitate out of water.
Magnesium carbonate + Lime → Calcium carbonate + Magnesium hydroxide
MgCO3 + Ca(OH)2→ CaCO3 + Mg(OH)2
Removal of Non-carbonate Hardness
In many cases, only the carbonate hardness needs to be removed, requiring only the addition of lime. However, if noncarbonate hardness also needs to be removed from water, then soda ash must be added to the water along with lime.
Each noncarbonate hardness compound will have a slightly different reaction. Here, we will consider the reactions of magnesium sulfate. The lime first reacts with the magnesium sulfate, as shown below:
Magnesium sulfate + Lime → Magnesium hydroxide + Calcium sulfate
MgSO4 + Ca(OH)2→ Mg(OH)2 + CaSO4
The resulting compounds are magnesium hydroxide, which will precipitate out of solution, and calcium sulfate. The calcium sulfate then reacts with soda ash:
Calcium sulfate + Soda Ash → Calcium carbonate + Sodium sulfate
CaSO4 + Na2CO3→ CaCO3 + Na2SO4
The calcium carbonate resulting from this reaction will settle out of the water. The sodium sulfate is not a hardness-causing compound, so it can remain in the water without causing problems.
Recarbonation
The reactions which remove carbonate and noncarbonate hardness from water require a high pH and produce water with a high concentration of dissolved lime and calcium carbonate. If allowed to enter the distribution system in this state, the high pH would cause corrosion of pipes and the excess calcium carbonate would precipitate out, causing scale. So the water must be recarbonated, which is the process of stabilizing the water by lowering the pH and precipitating out excess lime and calcium carbonate.
The goal of recarbonation is to produce stable water, which is water in chemical balance, containing the concentration of calcium carbonate in which it will neither tend to precipitate out of the water (causing scale) nor dissolve into the water (causing corrosion.) This goal is usually achieved by pumping carbon dioxide into the water. Excess lime reacts with carbon dioxide in the reaction shown below, producing calcium carbonate:
Lime + Carbon dioxide → Calcium carbonate + Water
Ca(OH)2 + CO2→ CaCO3 + H2O
Recarbonation also lowers the pH, which encourages the precipitation of calcium carbonate and magnesium hydroxide.
Recarbonation may occur in one step, in which the pH is lowered to about 10.4 and carbonate hardness is precipitated out. In some cases, a second recarbonation step is used to lower the pH to 9.8 and encourage yet more precipitation. In either case, the process must be carefully controlled since carbon dioxide can react with calcium carbonate and draw it back into solution as calcium bicarbonate, negating the softening process.

Alternatively, recarbonation can be achieved through the addition of acids such as sulfuric or hydrochloric acids or through polyphosphate addition. These types of recarbonation work differently from carbon dioxide addition.
In The Treatment Process
Equipment Used
As mentioned previously, lime softening uses the equipment already found in most treatment plants for turbidity removal. An overview of the lime treatment process is shown below.
Lime Softening Water Treatment
Sludge
Lime softening produces large quantities of sludge. In fact, for every pound of lime used, about two pounds of sludge are formed.
The softening process usually requires two sedimentation basins, each with a detention time of 1.5 to 3 hours, to deal with the large quantities of sludge. One sedimentation basin handles the sludge resulting from lime and soda ash softening and the other sedimentation basin deals with the sludge resulting from recarbonation.
Sludge may be disposed of through any number of methods. Landfill disposal is the most common, although sludge may sometimes be sent to sanitary sewers. Lime sludge has a high pH and has increasingly been disposed of by applying it to agricultural land to increase the pH of acidic soils.
Monitoring
If softening problems are discovered, the cause usually lies in either chemical feeder malfunctions or source water quality changes. A variety of water characteristics can influence lime-soda ash softening:
o Water hardness will determine the quantity of chemicals which must be added to soften the water.
o pH influences the chemical reactions in the softening process. A higher pH makes the process more efficient.
o Alkalinity determines whether the hardness in the water is carbonate or noncarbonate hardness.
o Temperature influences the rate of the reaction and the amount of hardness which the water will hold.
These four water characteristics should be monitored carefully when softening water using lime. In addition, coagulants used to remove turbidity can influence the alkalinity or pH of the water, thus affecting the softening process. After softening, the Langelier Index of the water should be tested to ensure that the water is not corrosive. We will study the Langelier index and corrosive water in more depth later. All three credit bureaus free.
Softening is especially well-suited to treating groundwater since groundwater characteristics tend to remain relatively constant. Changing water conditions require a great deal of manipulating the softening process to keep it efficient. In addition, the high turbidity found in surface water sometimes requires presedimentation prior to softening.
Chemicals Used in Lime Softening
Types of Lime
The lime used for softening comes in two forms – hydrated lime and quicklime. Both types of lime soften water in the same way, but the equipment required for the two types of lime is different.
Hydrated lime (Ca(OH)2) is also known as calcium hydroxide or slaked lime. Hydrated lime can be added to water as it is without requiring any special equipment, so it is a popular choice for small water treatment plants.
In contrast, quicklime (CaO), also known as calcium oxide or unslaked lime, must be slaked before it is used. Slaking is the process of converting quicklime to hydrated lime by adding water, as shown below:
Calcium oxide + Water → Hydrated lime
CaO + H2O → Ca (OH)2
Slaking requires specialized equipment. The cost of equipment and the operator time required to run the equipment usually make quicklime use uneconomical in small plants. However, since the chemical cost of quicklime is less than the cost of hydrated lime, quicklime is often used in large plants.
The slaking process can also allow a large plant to reuse a large quantity of the lime sludge produced in the softening process. First, the sludge is heated, and the calcium carbonate in the sludge produces calcium oxide:
Calcium carbonate → Calcium oxide + Carbon dioxide
CaCO3→ CaO + CO2
Then the calcium oxide can be slaked and reused in the plant. Reusing lime sludge cuts down on both chemical purchase and sludge disposal costs.
Lime Handling and Storage
Operators should observe safety procedures while handling both hydrated lime and quicklime. Lime dust can be harmful when it comes in contact with the eyes, nose, or mouth, and skin contact can cause burns. As a result, operators should wear goggles and dust masks as well as protective clothing.
Both hydrated lime and quicklime can deteriorate in quality over time while in storage. In addition, storing quicklime can cause safety problems. If quicklime comes in contact with water, it begins to slake, a process which produces a great deal of heat and can cause explosions when uncontrolled. Quicklime should never be stored with alum since the quicklime will absorb water away from the alum and cause an explosion.
Soda Ash
Soda ash (Na2CO3) comes in only one form and does not require any treatment before it is added to the water. Safety issues resemble those for lime handling. Soda ash dust irritates the eyes and mucous membranes of the nose, so the operator should wear protective clothing, goggles, and a dust mask. In addition, areas in which soda ash is used should be equipped with a ventilation system to deal with the dust.
Caustic Soda
Caustic soda (NaOH), also known as sodium hydroxide, can replace soda ash and some of the lime in the treatment process. The treatment process using caustic soda follows the same steps as that of lime-soda ash softening.
First, carbon dioxide reacts with the caustic soda to make sodium carbonate and water.
Carbon dioxide + Caustic soda → Sodium Carbonate + Water
Lime Softening Process
CO2 + 2NaOH → Na2CO3 + H2O

Then the remaining caustic soda can react with calcium bicarbonate and magnesium bicarbonate.
Calcium bicarbonate + Caustic soda → Calcium carbonate + Soda ash + Water
Ca(HCO3)2 + 2NaOH → CaCO3 + Na2CO3 + 2H2O
Magnesium bicarbonate + Caustic soda → Magnesium hydroxide + Soda ash + Water
Mg(HCO3)2 + 4NaOH → Mg(OH)2 + 2Na2CO3 + 2H2O

The caustic soda can also react with magnesium noncarbonate hardness, as shown below. Also note that the reactions between caustic soda and carbonate hardness produced soda ash, which can react with noncarbonate hardness as well.
Magnesium sulfate + Caustic soda → Magnesium hydroxide + Sodium sulfate
MgSO4 + 2NaOH → Mg(OH)2 + Na2SO4
Caustic soda has the advantages of stability in storage, lower sludge formation, and easy handling. However, safety issues still apply. Caustic soda is dangerous to the operator and can cause severe burns to the skin. As a result, rubber gloves, dusk masks, goggles, and a rubber apron should be worn while handling the chemical.
Ion Exchange Softening
Introduction
Softener Design
The other method commonly used for water softening is ion exchange softening, also known as zeolite softening. Ion exchange softening exchanges calcium and magnesium ions in water for sodium ions as the hard water passes through a softener. The softener is similar in design to a pressure filter, with resins in place of the filter media.
Ion exchange softener
During treatment, water enters the softener and is directed by a baffle. The water passes through a bed of resin underlain by a bed of gravel, then is collected by an underdrain and piped out of the softener.
Softener Operation
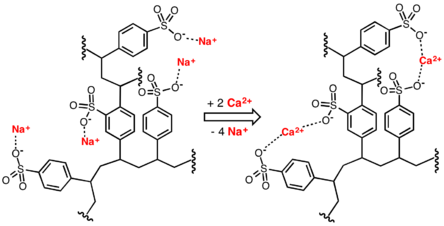
Despite the superficial resemblance between softeners and filters, the two operate in very different manners. In a softener, the bed is made up of resins, which are insoluble solids with attached cations or anions capable of reversible exchange with mobile ions of the opposite sign in the solutions in which they are brought in contact.
In the case of the ion exchange resins used in softening, sodium ions are attached to the insoluble solids of the resins. When water passes through the softener, the sodium ions are exchanged for calcium and magnesium ions in the water. The calcium and magnesium ions are retained on the resin grains. The water leaving the softener has sodium ions in the place of calcium and magnesium ions in its compounds, as shown below.
Since sodium ions do not cause hardness, the treated water is no longer hard.
In a properly operating softener, the treated water will have a hardness of 0 mg/L. To prevent corrosion due to excessively soft water, some of the source water is bypassed and does not pass through the softener. This untreated water is blended back into with the water downstream of the softener using a valve and meter to ensure that the proper quantity of water bypasses the softener.
There are many types of resins which can be used in ion exchange units. One type of resin commonly used in water softening is zeolite resin, which is why ion exchange softening is often known as zeolite softening. When other types of resins are used, ion exchange units can be used to remove minerals in brackish water or to remove alkalinity from water.
History
A scientific understanding of ion exchange did not begin until about 1850 when two English agricultural chemists, Harris S. Thompson and John T. Way , observed an exchange between ammonium and calcium in the soil. Twenty-six years later, Lemberg identified the resins involved and showed that the exchange of cations was reversible. It was soon discovered that various natural minerals, called zeolites, had exchange characteristics suitable for the softening of water.
In 1904, a German chemist by the name of Gans produced the first artificial resins. These artificial zeolites had effective capacities two to three times those of the naturally occurring minerals. Synthesis of these zeolites stimulated the industrial development of water softening. Later developments of other types of resins expanded the use of ion exchange to include the removal of other substances from water.
Softener Cleaning
Introduction
When all of the sodium ions on the softener resin have been replaced by calcium and magnesium ions, the softener must be recharged. The recharge process involves backwash, followed by regeneration, and ending in rinse.
Backwashing
Backwashing the softener is very similar to backwashing a pressure filter and the purpose is the same. Although the purpose of a softener is not to filter out particulate matter, some particles inevitably get caught in the softener. By sending water backwards through the softener, this particulate matter is removed.
Regeneration
After backwashing, the softener is ready to be regenerated. This is the part of the process in which the magnesium and calcium ions on the resin become replaced with sodium so that the softener can be used to treat more hard water. In order to regenerate the resin, a salt solution, known as brine, is allowed to flow through the softener for about an hour.
The salt used to regenerate the resin is ordinary table salt (sodium chloride, NaCl), so it is easy to handle. When dissolved in water, the salt dissociates into its constituent ions – Na+ and Cl.- The sodium ions replace the calcium and magnesium ions on the resin in the following manner (where “R” preceding an ion means that the ion is bound to the resin.)
RCa + NaCl → RNa + CaCl2
RMg + NaCl → RNa + MgCl2
So the regeneration process can be summarized as follows:
During regeneration, calcium chloride, magnesium chloride, and excess sodium chloride flow to waste.
Rinse and Waste Disposal
After the brine has been given a sufficient contact time, it must be rinsed out of the softener. During the rinse cycle, fresh water is passed through the unit as it would be during treatment, but with the effluent going to waste. Rinse usually takes about 20 to 40 minutes.
Both the spent brine from regeneration and from the rinse must be disposed of carefully since the calcium, magnesium, and sodium salts are corrosive and toxic to the environment. Spent brine is sometimes discharged in sewers or into streams at very high dilutions. Alternatively, the brine can be disposed of in a landfill.
In the Treatment Process
Location in the Plant
Ion exchange softening requires influent water free of turbidity since particulate matter can clog the resin bed. As a result, it is often one of the last steps in the treatment process, following flash mixing, flocculation, sedimentation, and filtration. As with lime-soda ash softening, ion exchange softening can be problematic when dealing with surface water with changing water characteristics.
Ion exchange softening is used widely in small water treatment plants and in individual homes. The process has many advantages for these installations, including its compactness, simplicity, and low cost. Operation of the unit can be nearly automatic and the chemicals used are relatively safe and easy to handle.
Monitoring
Like any other treatment process, ion exchange softening requires a certain degree of water quality monitoring. The following water quality characteristics should be tested:
• Hardness of influent water influences the time between cleanings. The more calcium and magnesium compounds are present in the water, the more quickly the sodium bound to the resin becomes used up and the more often the softener must be regenerated.
• Iron and manganese in the influent water will be caught in the filter bed and can plug it up. Iron and manganese should always be removed before water is softened.
• Sodium in the influent water will react with the softener’s resin as it would during regeneration, resulting in magnesium and calcium leaking through into the treated water.
• Chlorine residual of the influent water should be monitored since an excessive chlorine residual can damage the resin.
• Langelier Index should be monitored in the effluent to ensure that corrosive water is not being released to the distribution system.
Efficiency
In addition to raw water characteristics, the operation of the softener can influence efficiency. The most important factors are resin, flow, and cleaning.
Both the resin type and depth influence softening. There are many types of resins, each with different removal abilities, so the type of resin used will determine what can be removed from water. The depth of the resin bed will influence how much hardness can be removed from the water, with deeper beds removing more hardness.
Operation of the softener during the softening phase can also influence efficiency. Specifically, the flow rate of the water through the softener influences how much hardness is removed.
The efficiency of regeneration greatly affects operation of the softener during the softening phase. The salt dosage during regeneration, the brine concentration, and the brine contact time all influence how well sodium is regenerated on the resin surface. If regeneration is not complete, then the softener will not operate as long before it requires another round of cleaning.
Review
Hard water is caused by divalent cations, most commonly calcium and magnesium. If these cations combine with alkalinity, they produce carbonate hardness. If combined with cations other than alkalinity, they produce noncarbonate hardness.
Water can be softened using chemical precipitation (lime softening), ion exchange (zeolite softening), reverse-osmosis softening, electrodialysis, distillation, or freezing. After treatment, softened water should have a hardness of 80 to 90 mg/L as calcium carbonate. Softening processes can improve other water characteristics as well as lowering hardness, but they can also interfere with some treatment processes and can cause waste disposal problems.
Lime softening consists of raising the water’s pH to produce calcium carbonate and magnesium hydroxide which will settle out of the water. After softening, the water must be recarbonated to stabilize the water. Lime softening occurs in the flash mixer, flocculation basin, and sedimentation basin(s). Chemicals used in lime softening include some combination of hydrated lime, quicklime, soda ash, and caustic soda, all of which require safety procedures during handling.
Ion exchange softening occurs in a softener, which is typically found downstream from filtration. Magnesium and calcium ions in the water are replaced with sodium ions from the softener’s resin. After the sodium ions in the resin are exhausted, the softener must be cleaned by backwashing, regeneration, and rinsing.
References
Kerri, K.D. 2002. Water Treatment Plant Operation. California State University : Sacramento .
Minnesota Rural Water Association. Ion-Exchange (Zeolite) Softening.
Letterman, R.D., ed. 1999. Water Quality and Treatment: A Handbook of Community Water Supplies. McGraw-Hill, Inc.: New York .
Parks, C.S., and I.M. Abrams. Fundamentals of Ion Exchange in Water Treatment. Diamond Shamrock Corporation.
Ragsdale and Associates. Version III. New Mexico Water Systems Operator Certification Study Guide. NMED Surface Water Quality Bureau: Santa Fe .
National Drinking Water Clearing House. 1998. Lime Softening.
Assignment
1. What is hard water?
2. What are some of the problems associated with hard water?
3. Besides calcium and magnesium what other dissolved metals can cause hardness?
4. Explain the difference between carbonate and noncarbonate hardness.
5. Explain the chemical precipitation process in detail. Explain its disadvantages as well.
6. Explain the ion exchange softening process in detail. Explain its disadvantages as well.
7. What are the advantages of softening?
8. What are the disadvantages of softening?
9. What are the water characteristics that can influence lime-soda ash softening and how do they affect it?
10. During the ion exchange softening process, what water characteristics need to be monitored and how do they affect it?
POTABLE WATER
Drinking water or potable water is water safe enough to be consumed by humans or used with low risk of immediate or long term harm. In most developed countries, the water supplied to households, commerce and industry meets drinking water standards, even though only a very small proportion is actually consumed or used in food preparation. Typical uses (for other than potable purposes) include toilet flushing, washing and landscape irrigation. The word potable came into English from the Late Latin potabilis meaning drinkable.
Over large parts of the world, humans have inadequate access to potable water and use sources contaminated with disease vectors, pathogens or unacceptable levels of toxins or suspended solids. Drinking or using such water in food preparation leads to widespread acute and chronic illnesses and is a major cause of death and misery in many countries. Reduction of waterborne diseases is a major public health goal in developing countries.
Requirements:
In terms of mineral nutrients intake, it is unclear what the drinking water contribution is. Inorganic minerals generally enter surface water and ground water via storm water runoff or through the Earth’s crust. Treatment processes also lead to the presence of some minerals. Examples include calcium, zinc, manganese, phosphate, fluoride and sodium compounds. Water generated from the biochemical metabolism of nutrients provides a significant proportion of the daily water requirements for some arthropods and desert animals, but provides only a small fraction of a human’s necessary intake. There are a variety of trace elements present in virtually all potable water, some of which play a role in metabolism. For example sodium, potassium and chloride are common chemicals found in small quantities in most waters, and these elements play a role in body metabolism. Other elements such as fluoride, while beneficial in low concentrations, can cause dental problems and other issues when present at high levels.
Profuse sweating can increase the need for electrolyte (salt) replacement. Water intoxication (which results in hyponatremia), the process of consuming too much water too quickly, can be fatal
Water Resources:
Although covering some 70% of the Earth’s surface, most water is saline. Freshwater is available in almost all populated areas of the earth, although it may be expensive and the supply may not always be sustainable. Sources where water may be obtained include:
• ground sources such as groundwater, hyporheic zones and aquifers.
• precipitation which includes rain, hail, snow, fog, etc.
• surface water such as rivers, streams, glaciers
• biological sources such as plants.
• the sea through desalination
• water supply network
Spring water is groundwater that rises to the ground surface. Springs are often used as sources for bottled waters. Tap water, delivered by domestic water systems in developed nations, refers to water piped to homes and delivered to a tap or spigot. For these water sources to be consumed safely they must receive adequate treatment and meet drinking water regulations.
The most efficient way to transport and deliver potable water is through pipes. Plumbing can require significant capital investment. Some systems suffer high operating costs. The cost to replace the deteriorating water and sanitation infrastructure of industrialized countries may be as high as $200 billion a year. Leakage of untreated and treated water from pipes reduces access to water. Leakage rates of 50% are not uncommon in urban systems.
Because of the high initial investments, many less wealthy nations cannot afford to develop or sustain appropriate infrastructure, and as a consequence people in these areas may spend a correspondingly higher fraction of their income on water. 2003 statistics from El Salvador, for example, indicate that the poorest 20% of households spend more than 10% of their total income on water. In the United Kingdom authorities define spending of more than 3% of one’s income on water as a hardship.
Improving Availability:
Solar water disinfection is a low-cost method of purifying water that can often be implemented with locally available materials. Unlike methods that rely on firewood, it has low impact on the environment.
One organisation working to improve the availability of safe drinking water in some the world’s poorest countries is Water Aid International. Operating in 26 countries, WaterAid is working to make lasting improvements to peoples’ quality of life by providing long-term sustainable access to clean water in countries such as Nepal, Tanzania, Ghana and India. It also works to educate people about sanitation and hygiene.
Water Quality & Contamination:
Parameters for drinking water quality typically fall under three categories:
• physical
• chemical
• microbiological.
Physical and chemical parameters include heavy metals, trace organic compounds, total suspended solids (TSS), and turbidity.
Microbiological parameters include Coliform bacteria, E. coli, and specific pathogenic species of bacteria (such as cholera-causing Vibrio cholerae), viruses, and protozoan parasites.
Chemical parameters tend to pose more of a chronic health risk through buildup of heavy metals although some components like nitrates/nitrites and arsenic can have a more immediate impact. Physical parameters affect the aesthetics and taste of the drinking water and may complicate the removal of microbial pathogens.
Originally, fecal contamination was determined with the presence of coliform bacteria, a convenient marker for a class of harmful fecal pathogens. The presence of fecal coliforms (like E. Coli) serves as an indication of contamination by sewage. Additional contaminants include protozoan oocysts such as Cryptosporidium sp., Giardia lamblia, Legionella, and viruses (enteric). Microbial pathogenic parameters are typically of greatest concern because of their immediate health risk.
Throughout most of the world, the most common contamination of raw water sources is from human sewage and in particular human faecal pathogens and parasites. In 2006, waterborne diseases were estimated to cause 1.8 million deaths each year while about 1.1 billion people lacked proper drinking water. It is clear that people in the developing world need to have access to good quality water in sufficient quantity, water purification technology and availability and distribution systems for water. In many parts of the world the only sources of water are from small streams often directly contaminated by sewage.
Water treatment
Most water requires some type of treatment before use, even water from deep wells or springs. The extent of treatment depends on the source of the water. Appropriate technology options in water treatment include both community-scale and household-scale point-of-use (POU) designs.
Over the past decade, an increasing number of field-based studies have been undertaken to determine the success of POU measures in reducing waterborne disease. The ability of POU options to reduce disease is a function of both their ability to remove microbial pathogens if properly applied and such social factors as ease of use and cultural appropriateness. Technologies may generate more (or less) health benefit than their lab-based microbial removal performance would suggest.
The current priority of the proponents of POU treatment is to reach large numbers of low-income households on a sustainable basis. Few POU measures have reached significant scale thus far, but efforts to promote and commercially distribute these products to the world’s poor have only been under way for a few years.
In emergency situations when conventional treatment systems have been compromised, water borne pathogens may be killed or inactivated by boiling but this requires abundant sources of fuel, and can be very onerous on consumers, especially where it is difficult to store boiled water in sterile conditions and is not a reliable way to kill some encysted parasites such as Cryptosporidium or the bacterium Clostridium. Other techniques, such as filtration, chemical disinfection, and exposure to ultraviolet radiation (including solar UV) have been demonstrated in an array of randomized control trials to significantly reduce levels of water-borne disease among users in low-income countries, but these suffer from the same problems as boiling methods.
References:
1. WHO and UNICEF JMP website homepage, WHO, Geneva and UNICEF, New York, accessed on June 10, 2012
2. World Water Assessment Program, accessed on February 27, 2010
3. WHO and UNICEF Progress on Drinking-water and Sanitation: 2012 Update, WHO, Geneva and UNICEF, New York
4. Greenhalgh, Alison (March 2001). “Healthy living – Water”. BBC Health. Retrieved 2007-02-19.
